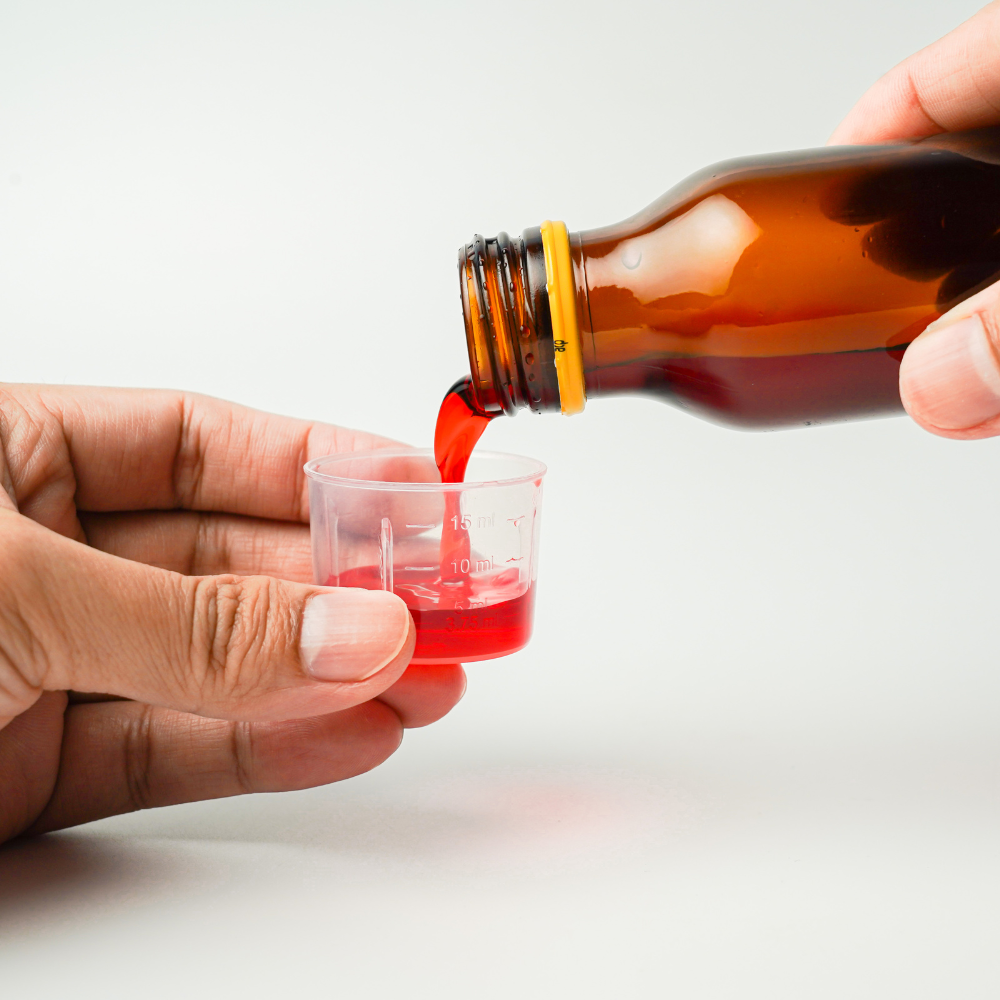
After the Maiden Therapeutics fiasco, the World Health Organization issued a product alert against another Indian pharmaceutical manufacturer. This time, the WHO has called out Marion Biotech Private Limited for its cough syrup formulations which were distributed in Uzbekistan. According to the WHO, two cough syrup formulations, AMBRONOL syrup, and DOK-1 Max syrup were found to be of substandard quality. The WHO said that these substandard formulations were reported to the agency on December 22. Since then Marion Biotech Private Limited has not provided any explanation to the WHO regarding its stance on the situation. Because of this, the organization has decided to issue a product alert warning against these two products and has advised people to not consume them.
Marion Biotech Private Limited is an Indian pharmaceutical company that specializes in manufacturing generic formulations and supplies them overseas. The company is located in Uttar Pradesh, India, as referenced by the WHO. AMBRONOL syrup and DOK-1 Max syrup, both of these products were suspected by the local healthcare authorities of being contaminated with harmful ingredients. Hence both of these syrups were then taken to the national quality control laboratories under the Ministry of Health of the Republic of Uzbekistan for further analysis. After a thorough analysis, national laboratories reported that the AMBRONOL syrup and DOK-1 Max syrup contained unhealthy amounts of diethylene glycol and ethylene glycol. This is similar to the case of another Indian pharmaceutical company, Maiden Therapeutics which caused the deaths of over 60 children in Gambia. Back in October, Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup, four of these cough syrup formulations manufactured by Maiden Therapeutics contained unhealthy levels of diethylene glycol and ethylene glycol which resulted into acute kidney failure in children of Gambia, resulting into their death. However, the investigation probe launched by the Indian government found that there was no such contamination found with the samples which Maiden Therapeutics had sent to Gambia.
Diethylene glycol and ethylene glycol are extremely harmful chemical substances that can cause serious illness in adults and can be fatal for children. Hence, finding such chemicals in harmful amounts in formulations developed mainly for children is an extremely alarming situation. Generally, cough syrups are prepared using glycerin or glycerol as a commonly used ingredient. Glycerin is used in cough syrups to increase the thickness or as a solvent . But because of its high price, many nefarious drug producers opt for Diethylene glycol, which is a cheaper alternative to the glycerin used in the industry. Although DEG or Diethylene glycol is not that much toxic for adults, it is still critically toxic to children. However, ethylene glycol is more toxic than diethylene glycol and can cause more harm to children. It can certainly damage body organs such as the liver, kidneys, and lungs, which was seen in Gambia as the children suffered from acute kidney failure. The WHO is looking at the marketing data of these two products to identify the countries in which these products were supplied other than Uzbekistan.